
Pioneering Neuromodulation Research with tVNS®
Discover why tVNS® is the preferred device for research on transcutaneous vagus nerve stimulation.With its precise cymba concha stimulation and wireless parameter flexibility, tVNS offers cutting-edge technology and unparalleled flexibility to explore new therapeutic protocols.Approved under the EU-MDR and backed by CB reports, our tVNS device ensures adherence to the highest standards of safety and efficacy in medical research.
tVNS® R for Research
Transcutaneous Vagus Nerve Stimulation Device for Research
-
Full Wireless Parameter Programmability
-
Patient App for Data Collection
-
Support for Clinical Trials
-
Triggered tVNS®
-
Legacy and Hook Electrode
-
Sham Stimulation
tVNS® Devices are Trusted by Leading Institutions Worldwide

































Why We Lead in Vagus Nerve Stimulation?
For over 20 years, our commitment to innovation has positioned us at the forefront of non-invasive vagus nerve stimulation.
Our unmatched research and development efforts are backed by solid clinical trials, ensuring our devices are not only effective but also safe and reliable. As an approved Class IIa medical device under the EU-MDR, we offer scientifically validated solutions in a market crowded with unverified alternatives.
Since introducing the Cerbomed Nemos® device, we’ve led the market in non-invasive vagus nerve technology. Our pioneering approach and sustained innovation keep us ahead, with our devices being preferred by healthcare professionals and researchers worldwide due to unmatched safety, reliability and precision.
Developed and manufactured in Germany, our devices meet the highest quality standards, reflecting our dedication to excellence and the well-being of our patients.
Why Choose our tVNS® Devices for Research?
Our tVNS® device is the only non-invasive vagus nerve stimulator that is approved as a Class IIa medical device under the European Medical Device Regulation (EU-MDR). tVNS® sets itself apart with its non-invasive, clinically validated approach, offering a safer and more comfortable alternative to invasive methods. Uniquely targeting the cymba concha for 100% innervation of the auricular branch of the vagus nerve (ABVN) with our patented electrode, tVNS® ensures maximum efficacy compared to other devices that only achieve partial innervation.
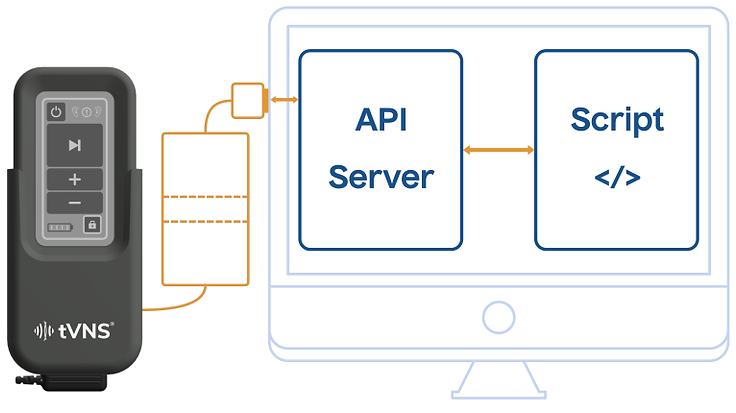
Introducing the tVNS® Triggerbox
Launching December 1, 2024, the tVNS® Triggerbox advances neuromodulation research by providing a cable-bound system that minimizes latencies and enhances protocol reproducibility. This key advancement ensures precise, reliable experimental setups for cutting-edge studies.

Approved Medical Device
Our tVNS® E device is the only non-invasive vagus nerve stimulation (VNS) device that is approved as a medical device under the EU-MDR. The supporting comprehensive CB reports not only highlight our device’s safety and efficacy but also facilitate smoother approval processes for your research protocols by ethics committees.



Cymba Concha Stimulation
Benefit from our device’s electrode design, targeting the cymba concha, a method preferred by researchers for its 100% innervation of the auricular branch of the vagus nerve (ABVN). This targeted approach is designed to maximize therapeutic outcomes by engaging the vagus nerve more effectively than other non-invasive methods.

Wireless Connectivity and Programmability
Leverage the power of wireless technology for seamless operation and data management. The tVNS® Research app enables remote programming of stimulation parameters, providing the flexibility needed to adapt to the evolving needs of complex research protocols without interrupting the participant’s experience.



Made in Germany
Trust in the quality and reliability of a device that’s proudly designed and manufactured in Germany. Our commitment to excellence guarantees that every tVNS® device meets the highest standards of performance, durability, and safety, making it the ideal choice for institutions demanding the best in research equipment.

Titanium-Iridium Electrode
Our tVNS® device features titanium-iridium electrodes, most often used in implants and known for their biocompatibility, offering optimal stimulation with minimal irritation. This superior choice sets us apart from stainless steel alternatives, ensuring a more comfortable and effective treatment experience.



_edited.jpg)
GDPR-Compliant Data Processing
With a commitment to privacy and security, our tVNS® system ensures that all data collected through the app is processed in strict adherence to the General Data Protection Regulation (GDPR). Researchers can collect, store, and analyze sensitive data with confidence, knowing that participant privacy is protected.



Global Network of Trusted Institutions
Join an esteemed community of researchers from leading universities and hospitals around the world who have chosen tVNS® for their studies in the global effort to advance neurological research and patient care.



20+ Years of Experience
With over 20 years of dedicated research and development, we proudly developed the world's first approved non-invasive vagus nerve stimulator, the Cerbomed Nemos® device. Our deep-rooted expertise and pioneering spirit have established us as leaders in the field, committed to delivering groundbreaking and effective solutions for our patients.

tVNS® for Research: An Overview
Welcome to the frontier of vagus nerve stimulation research. Our tVNS® R device is designed for flexibility and precision, offering full wireless programmability for comprehensive clinical studies.
With the tVNS® Research and tVNS® Patient apps, adjustment of stimulation parameters, data collection and patient feedback are seamlessly integrated, enhancing the scope and accuracy of your research.
We are committed to facilitating your clinical trials and provide support for studies, including a comprehensive CB safety report for ethics approvals and access to a global network of experts.
Explore the potential of tVNS® R to forge new paths in neurological research and patient care.
Full Wireless Parameter Programmability and Triggered tVNS®
With the tVNS® Research app, you have the capability to wirelessly adjust a comprehensive range of parameters including frequency, pulse width, current intensity, and stimulation intervals.
In addition, we offer a separate PC-based tVNS® manager software. This software enables reaction to outside trigger signals (e.g. ECG R-wave trigger generated by an external ECG system, transferred to a PC).
Thus, closed loop tVNS® or triggered tVNS® can be realized in a scientific setup.


Precision in Research with the tVNS® Triggerbox
The new tVNS® Triggerbox, launching December 1, 2024, marks a significant advancement in the field of tVNS® research.
Designed in response to feedback from scientific communities, this device addresses the crucial need for more stable and reproducible stimulation protocols in tVNS®.
By replacing Bluetooth connections with a cable-bound system, the tVNS® Triggerbox significantly reduces latencies and fluctuations, enabling precise synchronization with external trigger signals. This innovation not only enhances the reliability of experimental setups but also simplifies the integration with existing research frameworks, promising to push the boundaries of tVNS® research further.
Research and Patient App
Your tVNS® R device gives you access to two apps: the tVNS® Research and tVNS® Patient app. All technical parameters can easily be reprogrammed and wirelessly transmitted with the tVNS® Research app, allowing the researcher to try completely new stimulation protocols. In addition, you can collect and store technical and patient data.
The tVNS® Patient app automatically stores all technical data (stimulation parameters, time and duration of the stimulation etc.) and offers a questionnaire for patient feedback.
tVNS® Patient is fully compliant with the General Data Protection Regulation (GDPR), ensuring that patient data is handled with the utmost care and confidentiality.


Customization and Support for Trials
We offer an individual adaptation of all components of the device for certain studies, e.g by changing the questionnaire in order to allow better feedback from the patient, or through the implementation of special stimulation parameters or technical ideas.
The tVNS® R device is defined as a device for investigational use. We can provide a CB report that proves the safety according to all medical device standards, in order to assist you with ethics approvals. So far, it has been accepted by all ethics committees.
In addition, we offer an international expert network and can direct any questions to experienced people around the world.
Legacy and Hook Electrode
We offer two different types of electrode designs with our tVNS® devices:
The Hook Electrode (left) allows for better fixation and usability during active tasks. It also allows patients to wear earphones.
The Legacy Electrode (right) can be used if the patient has small ears or a special ear shape. The integrated slider allows for better adaption to the ear.


Sham and fMRI Electrode Options
The well-known sham-stimulation option at the earlobe is possible with our Legacy Electrode.
Upon request, we also provide more sophisticated sham-stimulation options, as well as fMRI-compatible electrodes.
Support for Clinical Trials
We offer an individual adaptation of all parts for certain studies, e.g by changing the questionnaire in order to allow better feedback from the patient, or through the implementation of special stimulation parameters or technical ideas.
The tVNS® R device is defined as a device for investigational use. We can provide a CB report that proves the safety according to all medical device standards, in order to assist you with ethics approvals. So far, it has been accepted by all ethics committees.
In addition, we offer an international expert network and can direct any questions to experienced people around the world.
.jpg)
Book a Personal Consultation with our Research Experts
Contact our tVNS® Research Team to learn more about tVNS® and receive personalized information and support, such as CB Reports and Instructions for Use (IFU).



.png)




