
Transform Patient Care with tVNS®
Empower your practice and patients with a non-invasive solution that enhances well-being without the need for medication.
With its precise cymba concha stimulation and wireless parameter flexibility, tVNS® offers cutting-edge technology and unparalleled flexibility to explore new therapeutic protocols.
Approved under as a Class IIa medical device under the EU-MDR, our tVNS® device ensures adherence to the highest standards of safety and efficacy in medical research.
tVNS® Devices are Trusted by Leading Institutions Worldwide
































tVNS® R for Research
Transcutaneous Vagus Nerve Stimulation Device for Research
-
Full Wireless Parameter Programmability
-
Patient App for Data Collection
-
Support for Clinical Trials
-
Triggered tVNS®
-
Legacy and Hook Electrode
-
Sham Stimulation
Key Benefits for Patients
Certified Medical Device
tVNS® is the world's only non-invasive vagus nerve stimulation approved as a Class IIa medical device under the EU-MDR, offering a scientifically validated treatment for conditions related to sympathovagal imbalance.
Non-Invasive Approach
A safe and gentle alternative to surgery and medication, tVNS® stimulates the vagus nerve through the skin, offering a harmonious path to wellbeing with very low side-effects.
Most Effective Stimulation Site
tVNS® is the only non-invasive stimulation device that stimulates the cymba concha, resulting in 100% vagus nerve innervation (as opposed to e.g. the tragus, which only results in 45% innervation).
Minimal Side
Effects
Transitioning away from traditional medications often aims to reduce side effects. tVNS®, as a drug-free therapy, offers a safer alternative with lower risk of long-term adverse effects.

Why We Lead in Vagus Nerve Stimulation?
For over 20 years, our commitment to innovation has positioned us at the forefront of non-invasive vagus nerve stimulation.
Our unmatched research and development efforts are backed by solid clinical trials, ensuring our devices are not only effective but also safe and reliable. As an approved Class IIa medical device under the EU-MDR, we offer scientifically validated solutions in a market crowded with unverified alternatives.
Since introducing the Cerbomed Nemos® device, we’ve led the market in non-invasive vagus nerve technology. Our pioneering approach and sustained innovation keep us ahead, with our devices being preferred by healthcare professionals and researchers worldwide due to unmatched safety, reliability and precision.
Developed and manufactured in Germany, our devices meet the highest quality standards, reflecting our dedication to excellence and the well-being of our patients.
What is tVNS®?
Elevating patient treatment options with non-invasive vagus nerve stimulation for the treatment of neurological diseases
When traditional pharmacological or invasive treatments fall short or present challenging side effects, tVNS® offers an effective and patient-friendly treatment option.
The vagus nerve, central to our body’s nervous system, plays a critical role in maintaining homeostasis. tVNS®, or Transcutaneous Vagus Nerve Stimulation, harnesses the therapeutic potential of this nerve through targeted stimulation of its auricular branch, accessible within the ear.
This innovative approach utilizes gentle electrical impulses to modulate the vagus nerve’s activity, encouraging a state of balance across the body’s systems. The result? A reduction in the symptoms of neurological diseases associated with sympathovagal imbalance, achieved through a method that avoids the invasiveness of surgery and the side effects associated with medication. Approved as a Class IIa medical device under the European Medical Device Regulation (MDR), tVNS® sets a new standard in the treatment of neurological conditions, offering a blend of efficacy, safety, and simplicity.

tVNS® is indicated to treat the following conditions:
✓Anxiety
✓Atrial fibrillation
✓Autism
✓Cognitive Impairment
✓Depression
✓Epilepsy
✓Irritable Bowel Syndrome
✓Inflammatory Bowel Disease
✓Migraines
✓Parkinson's Disease
✓Prader-Willi Syndrome
✓Sleep Disorders
✓Systemic Sclerosis
✓Tinnitus
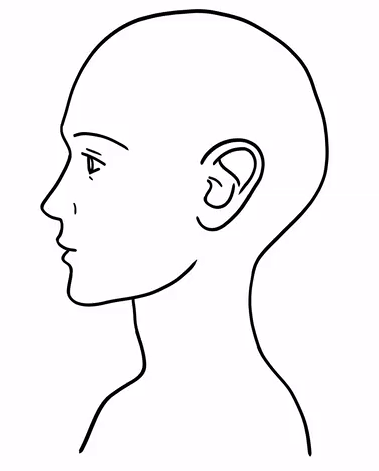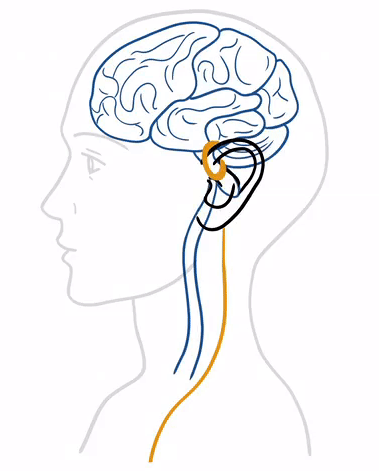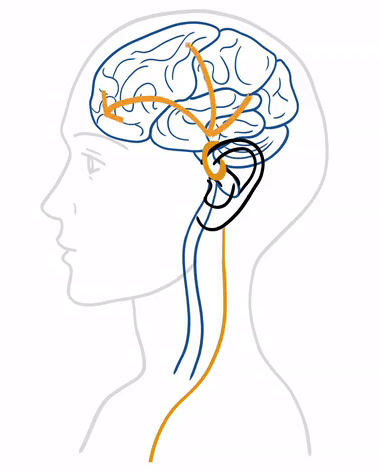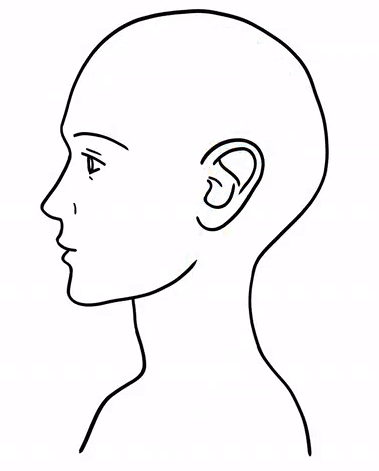

Treatment with tVNS® is characterized by its simplicity and adaptability to everyday life. At its core, tVNS® operates on the principle of neurostimulation, a process that engages the vagus nerve’s capacity to influence various bodily functions and systems. tVNS® stimulates the auricular branch of the vagus nerve (ABVN) through the skin with gentle and precise electrical impulses that propagate therapeutic signals to the brain, first to the brain stem and then to higher centers, aiming to correct dysregulation within the nervous system.
The application of tVNS® is designed with the patient in mind: it is easy, portable, and discreet, making it possible to incorporate treatment sessions into your schedule without interruption to your daily tasks. Whether you’re at home, in the office, or traveling, tVNS® provides a consistent and accessible means to manage symptoms of neurological disorders, including the reduction of seizure frequencies in epilepsy, among other conditions.
Each session with the tVNS® device is a step towards rebalancing the nervous system, leveraging the natural pathways of the body to foster healing and symptom relief. The device’s ease of use ensures that starting and maintaining a regimen with tVNS® is straightforward, empowering users to take control of their health in a non-intrusive, effective manner.
How does treatment with tVNS® work?
Patients can use tVNS® anytime, anywhere. It's easy to use, portable, and discrete.
Research and Patient App
Your tVNS® R device gives you access to two apps: the tVNS® Research and tVNS® Patient app. All technical parameters can easily be reprogrammed and wirelessly transmitted with the tVNS® Research app, allowing the researcher to try completely new stimulation protocols. In addition, you can collect and store technical and patient data.
The tVNS® Patient app automatically stores all technical data (stimulation parameters, time and duration of the stimulation etc.) and offers a questionnaire for patient feedback.
tVNS® Patient is fully compliant with the General Data Protection Regulation (GDPR), ensuring that patient data is handled with the utmost care and confidentiality.

Why choose our tVNS® devices?
Our tVNS® device is the only non-invasive vagus nerve stimulator that is approved as a Class IIa medical device under the European Medical Device Regulation (EU-MDR). tVNS® sets itself apart with its non-invasive, clinically validated approach, offering a safer and more comfortable alternative to invasive methods. Uniquely targeting the cymba concha for 100% innervation of the auricular branch of the vagus nerve (ABVN) with our patented electrode, tVNS® ensures maximum efficacy compared to other devices that only achieve partial innervation.

Approved Medical Device
Our tVNS® device is the only non-invasive vagus nerve stimulation (VNS) device that is approved as a medical device under the EU-MDR. It uniquely targets the Cymba Concha for 100% innervation of the auricular branch of the vagus nerve, unlike other devices that stimulate other areas and are not manufactured under the same quality and safety standards.


Non-Surgical Approach
tVNS® offers the efficacy of vagus nerve stimulation without the complexities or risks of surgical interventions. This means meaningful therapeutic benefits, all under your patient's control, without invasive procedures.

Minimal Side Effects
Transitioning away from traditional medications often comes with the pursuit of fewer side effects. tVNS®, being drug-free, ensures a therapeutic experience with reduced potential for long-term adverse outcomes, positioning itself as a safer alternative.



Made in Germany
Our commitment to excellence ensures that each device meets the highest standards of quality and performance, embodying the renowned precision of German engineering and manufacturing. With tVNS®, you’re choosing a device built on a foundation of durability and innovative design, trusted by healthcare professionals and researchers worldwide.


Innovative App Integration
Tracking and understanding your treatment progression is crucial. Our dedicated tVNS® Patient App not only provides real-time monitoring but also facilitates seamless sharing of data with healthcare professionals.



Remote Flexibility
In our modern age, the convenience of remote access is invaluable. tVNS® extends this by offering device delivery directly to your patient's doorstep and remote training. Your patient can initiate their treatment journey without the hassles or constraints of travel.

Easy to Use
Functionality should not compromise simplicity. The tVNS® device, while technologically advanced, is as intuitive as a smartphone, making integration into daily routines effortless.
Clinical Evidence:
The Science behind tVNS®
Discover the proven effectiveness and safety of tVNS®, highlighted by pivotal clinical trials and studies. Our unique focus on cymba concha stimulation, quality control and clinical evidence, positions tVNS® as the preferred choice among researchers, ensuring we lead in neurotherapeutic innovation and patient care.
.jpg)
Epilepsy
After a 20 week treatment period, active tVNS® patients recorded an average 34.2% reduction of seizure frequency.
Prader-Willi-Syndrome
After a treatment period of 12 months, 80% of tVNS® patients recorded a reduction of mean number of outbursts per day of 50%, compared to mean number of outburst per day before treatment.
Migraines
Treatment with tVNS® led to a reduction of 50% of the frequency of days with migraines after a treatment period of 12 weeks.
Depression
tVNS® led to a reduction of depression-specific HAMD score of minimum 2-3 points in adult patients when used in parallel with psychopharmacological and psychotherapy treatments after a period of 9 weeks.

tVNS Healing Stories
Take the Next Step in Patient Care with tVNS®
Discover the potential of tVNS® to transform the treatment landscape for your patients. Embrace a solution that offers a safe, non-invasive pathway to relief, especially for those who have explored conventional treatments without success. Join us in leading the way towards innovative, compassionate care. Connect with our qualified experts today to learn more about integrating tVNS® into your practice and how it can benefit your patients. Together, let’s pave the path to better health and improved quality of life.



.png)








